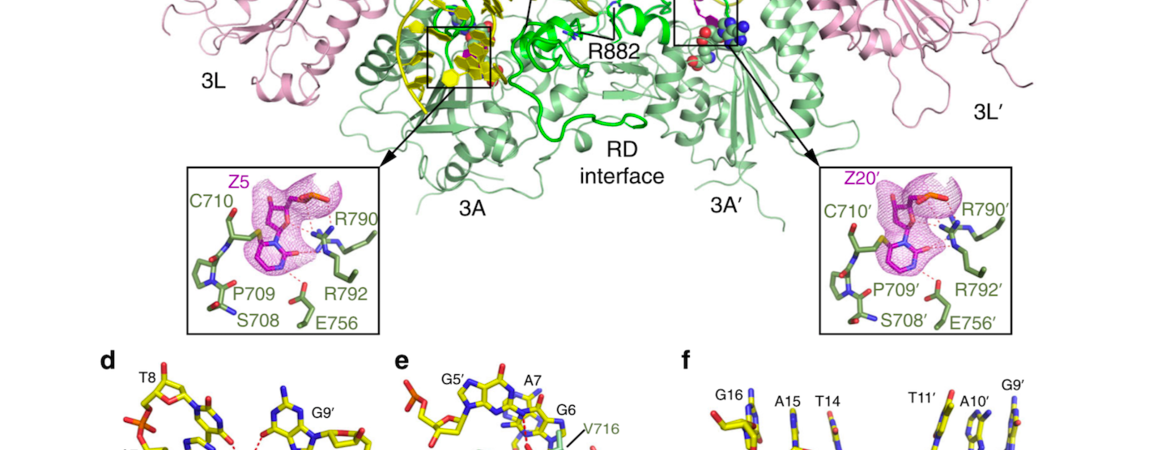
Jikui Song, an associate professor of biochemistry at UC Riverside, has published new work in Nature Communications that builds on his lab’s recent success in cracking the structure of DNMT3A, an enzyme that plays a key role in DNA methylation.
DNA methylation is a process involving the transfer of methyl groups to the DNA molecule, which critically influences gene expression, genomic stability and cell differentiation. Mutations of DNMT3A have been linked to a variety of diseases, such as acute myeloid leukemia, or AML, and overgrowth syndrome.
“DNMT3A mutations have been identified in about 25% AML patients; among these, the arginine-to-histidine mutation at site 882, or R882H, represents the most frequent one,” Song said. “The R882H mutation is associated with increased cell proliferation and correlates with poor clinical outcome. How this mutation impacts the function of DNMT3A and disease progression, however, has not been well understood.”
In the study, Song’s team solved the structures of DNMT3A that carrried the R882H mutation to identify the structural defects caused by this mutation. In addition, the team performed biochemical assays to understand how the R882H mutation affects the way DNMT3A works.
“Our study shows that this mutation affects the substrate recognition of DNMT3A in multiple ways,” Song said. “On the one hand, it reduces the activity of DNMT3A toward its native DNA substrates. On the other hand, this mutation also drives DNMT3A to act on non-native substrates. Our work, therefore, explains how this mutation impairs the function of DNMT3A in AML and other related diseases.”
Song, the study’s corresponding author, was joined by graduate student Hiwot Anteneh, the first author of the research paper, and postdoctoral researcher Jian Fang.
This study was funded by grants from the National Institutes of Health.
The research paper is titled, “Structural basis for impairment of DNA methylation by the DNMT3A R882H mutation.”